🌊 AMPHOTERIC COMPOUNDS
Amphoteric compounds are unique substances that can act as both acids and bases, depending on the nature of the substances they interact with. This dual behavior is fundamental in acid-base chemistry and is commonly observed in water and metal oxides.
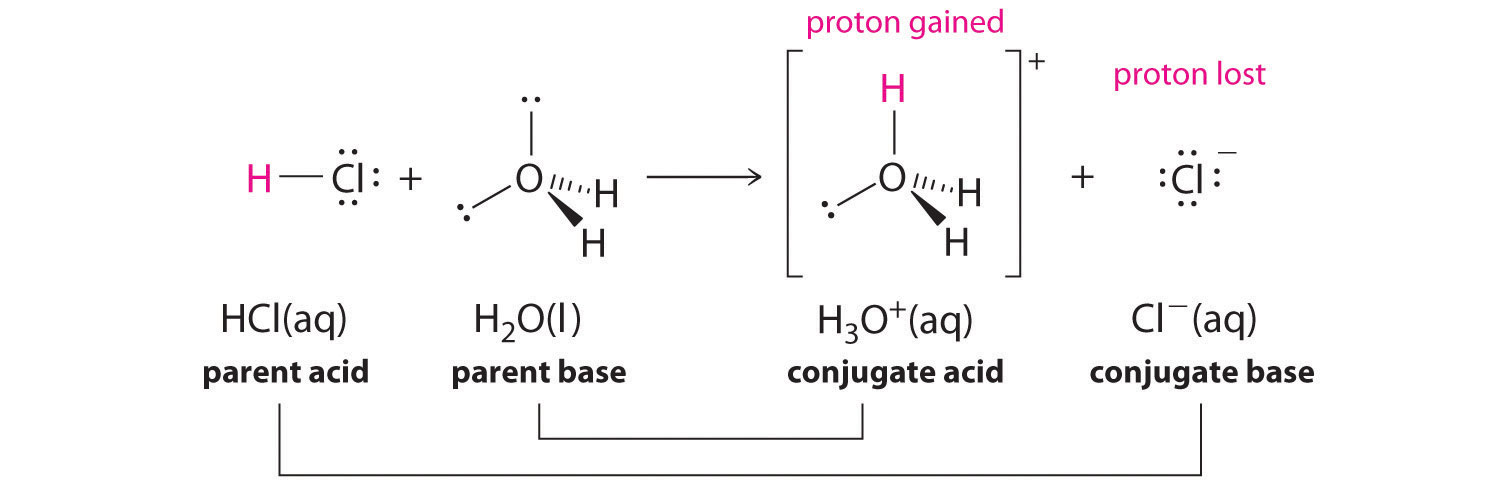
📘 Example 1: Water as a Base
Here, water acts as a base while HCl donates a proton, acting as an acid.
📗 Example 2: Water as an Acid
Here, water donates a proton and acts as an acid, while ammonia accepts it, acting as a base.
💡 Conclusion: Water behaves as both an acid and a base depending on the reaction partner, making it an excellent example of an amphoteric substance.
🔗 Other Useful Links
- News By Amurchem
- Free Web Development Course
- All-in-One Exam Prep Portal
- Articles by Amurchem
- Grade 12 Section
- Grade 11 Section
- Grade 10 Section
- Grade 09 Section
- Home and Online Tuition
- Labs By Amurchem
- Science Lectures By Amurchem
© 2025 AmurChem. All rights reserved.
Tags
acid-base reactions
amphoteric compounds
amphoteric substances examples
chemistry concepts
Federal Board Chemistry
Grade 10 Chemistry
National Curriculum Pakistan
water as acid and base