Group Trends of Halogens
Gradation of Characteristic Properties Within Group 17
Introduction
The elements of Group 17 in the periodic table are known as halogens (from Greek: "salt former"). These include: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At). Astatine is a rare, radioactive element with similar chemical properties. Halogens have 7 electrons in their outer shell and typically gain one electron to complete their octet.
Group Trends
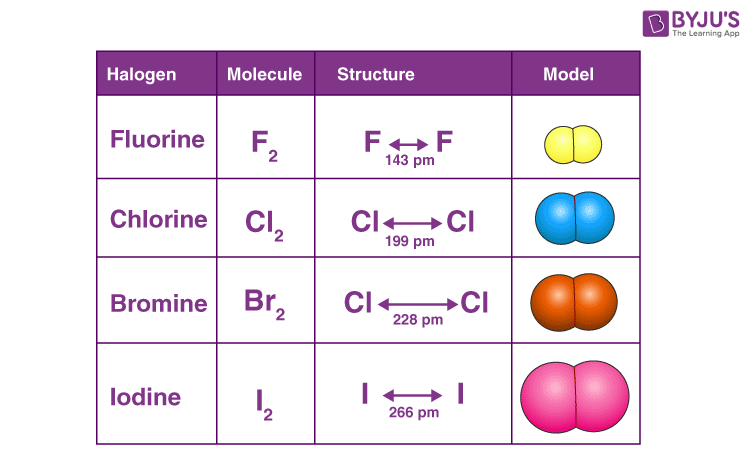
Atomicity and Physical States
Halogens exist as diatomic molecules (X2) with single covalent bonds. They are held together by Van der Waals forces, which increase with atomic size. At room temperature:
- F2 – Pale yellow gas
- Cl2 – Greenish gas
- Br2 – Reddish-brown liquid
- I2 – Shiny black solid
Electronegativity
Halogens are highly electronegative. Fluorine is the most electronegative element and forms hydrogen bonds in HF. Electronegativity decreases from fluorine to iodine:
Dissociation Energy
The bond dissociation energy of halogens follows:
Fluorine is an exception due to repulsion between lone pairs on small fluorine atoms, lowering its bond energy.
Reduction Potential
Halogens act as strong oxidizing agents. The oxidizing power decreases down the group:
Example of displacement:
Melting and Boiling Points
Both melting and boiling points increase from fluorine to iodine due to stronger intermolecular forces in larger molecules.
Oxidation States
Halogens typically show a -1 oxidation state when bonded with less electronegative elements. Fluorine, being the most electronegative, always shows a -1 state. Other halogens can show +1, +3, +5, and +7 in compounds with more electronegative atoms.
Reactivity
Halogens are highly reactive. Fluorine is the most reactive due to its high electronegativity and low bond dissociation energy. It can displace all other halogens from their salts.
Oxyacid Formation
Fluorine does not form oxyacids, but other halogens do. Types of halogen oxyacids:
- Hypohalous acids (HXO)
- Halous acids (HXO2)
- Halic acids (HXO3)
- Perhalic acids (HXO4)
🔗 Other Useful Links
- News By Amurchem
- Free Web Development Course
- All-in-One Exam Prep Portal
- Articles by Amurchem
- Grade 12 Section
- Grade 11 Section
- Grade 10 Section
- Grade 09 Section
- Home and Online Tuition
- Labs By Amurchem
- Science Lectures By Amurchem
© 2025 AmurChem. All rights reserved.





